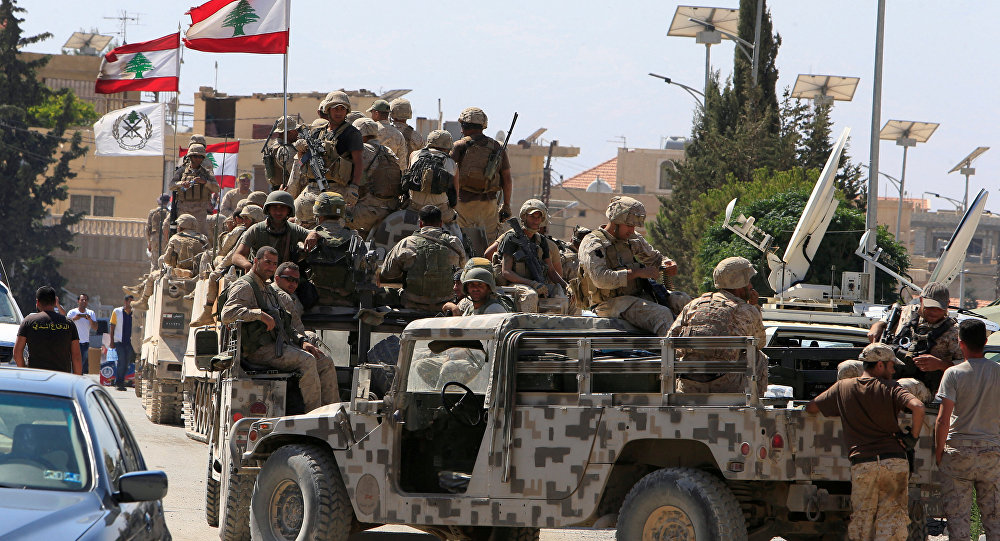
Explosions rock Tehran as US, Israel continue strikes - Video
00:30
| Home page Science |
|

The US Food and Drug Administration on Friday approved a highly anticipated new drug designed to slow cognitive decline in patients in mild and early stages of Alzheimer's disease.
Axar.az reports the FDA approval of the drug, Leqembi, also known as lecanemab, comes just days after the regulatory agency was harshly criticised in a congressional report for its green-lighting of another Alzheimer's drug, Aduhelm.
And it was granted despite trial results showing the monoclonal antibody treatment carries risks of brain swelling and bleeding.
Both drugs were approved through an accelerated process that allows the FDA to fast-track approval of drugs for serious conditions where there is an unmet medical need.
|
Date
2023.01.07 / 11:38
|
Author
Axar.az
|













.jpg)