Strong earthquake in Turkiye: There are ruins - Breaking
22:10
| Home page Health |
|
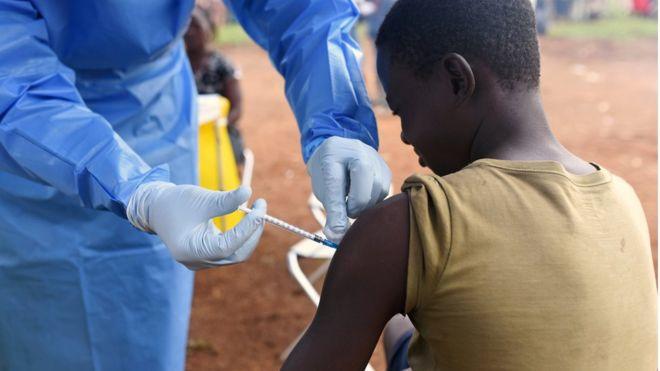
The US's Food and Drug Administration (FDA) has authorized the emergency use of the Ebola drug remdesivir for treating the coronavirus.
Axar.az reports citing foreign media that the authorization means the anti-viral drug can now be used on people who are hospitalised with severe Covid-19.
A recent clinical trial showed the drug helped shorten the recovery time for people who were seriously ill.
But emergency FDA authorisation is not the same as formal approval, which requires a higher level of review.
Experts have also warned the drug - which was originally developed to treat Ebola, and is produced by Gilead pharmaceutical company - shouldn't be seen as a "magic bullet" for coronavirus.
|
Date
2020.05.02 / 10:13
|
Author
Axar.az
|